Titration of sulfuric acid with sodium hydroxide
general remarks
Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences.
First of all, as sulfuric acid is diprotic, stoichiometry of the neutralization reaction is not 1:1, but 1:2 (1 mole of acid reacts with 2 moles of sodium hydroxide).
Second, as sulfuric acid is diprotic, we could expect titration curve with two plateaux and two end points. However, that's not the case. Even if the second dissociation constant is much lower than the first one (pKa1 = -3, pKa2 = 1.99), it is still high enough to not give its own inflection point, and titration curve looks almost identical to that of hydrochloric acid:
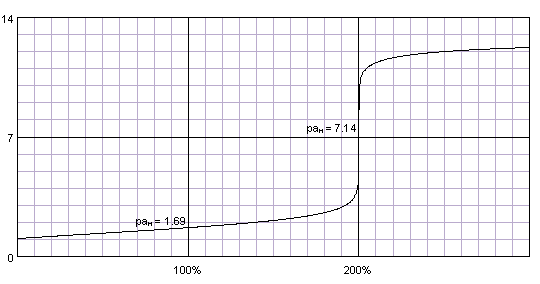
0.1 M sulfuric acid titrated with 0.1 M strong monoprotic base. Titration curve calculated with BATE - pH calculator.
reaction
This is a simple neutralization reaction:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
sample size
Depending on the titrant concentration (0.2 M or 0.1 M), and assuming 50 mL burette, aliquot taken for titration should contain about 0.34-0.44 g (0.17-0.23 g) of sulfuric acid (3.5-4.5 or 1.7-2.3 millimoles).
end point detection
Equivalence point of strong acid titration is usually listed as exactly 7.00. In the case of sulfuric acid second step of dissociation is not that strong, and end point is shifted up by tenths of the pH unit - but we are still very close to 7. Thus the best indicator of those listed on pH indicators preparation page is bromothymol blue. However, as we have discussed on the acid-base titration end point detection page, unless we are dealing with a diluted solution (in the range of 0.001 M) we can use almost any indicator that gives observable color change in the pH 4-10 range. In effect we can safely use the most popular phenolphthalein and titrate to the first visible color change.

Color change of phenolphthalein during titration - on the left, colorless solution before end point, on the right - pink solution after end point. Note we have to end titration at first sight of color change, before color gets saturated.
solutions used
To perform titration we will need titrant - 0.2 M or 0.1 M sodium hydroxide solution, indicator - phenolphthalein solution and some amount of distilled water to dilute hydrochloric acid sample.
procedure
- Pipette aliquot of sulfuric acid solution into 250mL Erlenmeyer flask.
- Dilute with distilled water to about 100 mL.
- Add 2-3 drops of phenolphthalein solution.
- Titrate with NaOH solution till the first color change.
result calculation
According to the reaction equation
H2SO4 + 2NaOH → Na2SO4 + 2H2O
sulfuric acid reacts with sodium hydroxide on the 1:2 basis. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used.
To calculate sulfuric acid solution concentration use EBAS - stoichiometry calculator. Download determination of sulfuric acid concentration reaction file, open it with the free trial version of the stoichiometry calculator.
Click button above NaOH in the input frame, enter volume and concentration of the titrant used. Click button. Read number of moles and mass of sulfuric acid in the titrated sample in the output frame. Click button in the output frame below sulfuric acid, enter volume of the pipetted sample, read sulfuric acid concentration.
sources of errors
Apart from general sources of titration errors, when titrating sulfuric acid we should pay special attention to titrant. Sodium hydroxide solutions are not stable as they tend to absorb atmospheric carbon dioxide. Sulfuric acid is much stronger than carbonic acid, so it will slowly expel carbon dioxide from the solution, but initially presence of carbonates will mean that to reach end point we need to add axcess of titrant.





