Precipitation titration end point detection
Compared to other types of titration - complexometric, potentiometric and acid base - precipitation titrations don't have a set of universal indicators, that you can select from when designing a new method. Each precipitation titration method has its own, specific way of end point detection. The closest to being universal are Fajans adsorption indicators, but even these are very limited in their applications.
Probably most popular precipitation titration - determination of chlorides by Mohr method - uses red silver chromate for the end point detection. At the beginning of the titration we add some small amount of CrO42- to the solution. As long as there are still chlorides present, Ag+ concentration is too low for the silver chromate to precipitate. After equivalence point Ag+ concentration goes up and chromate precipitates, making solution red.
This is an interesting case to discuss, as it is relatively easy to estimate necessary concentrations. We have two weakly soluble salts, silver chloride:
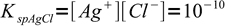 1
1and silver chromate
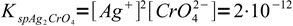 2
2Reaction taking place during titration is
Ag+ + Cl- → AgCl
At the equivalence point
 3
3At this Ag+ concentration we should start to see precipitating silver chromate. That means we need
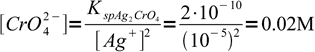 4
4This is a nice, easily calculated number, which has an unfortunate practical flaw. 0.02 M chromate solution is so strongly colored, that the red precipitate is not easily visible and the end point is hard to spot. Thus it is necessary to use lower concentration of chromate, and experiments show that concentration of about 0.004 M is acceptable - titrated solution is still yellowish, but the red color is hard to miss. However, as the chromate concentration is lower than optimal, question is, how much excess silver we have to add to the solution before we will be able to see the color change. This excess will mean a positive error in the determination.
If concentration of chromate is 0.004 M, concentration of Ag+ when the preipitation starts must be
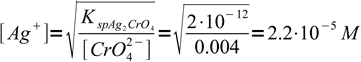 5
5That is 1.2 × 10-5 M more than the equivalence concentration. Assuming final volume of the titrated solution is about 100 mL and titrant concentration is about 0.1M, that means we need to add
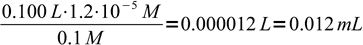 6
6excess of the Ag+ solution. This is well below errors from all other sources - please remember, that 50 mL class A burette has accuracy of 0.05 mL. In practice even smaller concentrations of chromate will still not change error much, besides, it is easy to check error with a blind test.
Note, that in the discussion above we have assumed no protonation of CrO42-. That's not necessarily correct - pKa2 for chromic acid is 6.5, so even in neutral solution substantial part of the acid is in the form of HCrO4-. Luckily, even at these lower concentrations of CrO42- errors are so small we can safely ignore them, as long as pH doesn't differ much from neutral.
However, due to the chromate protonation Mohr method will not work in the acidified solutions. That's where Volhard method comes handy. Again, this method uses a specific indicator. Volhard method is based on the back titration - we add known amount of the Ag+ to the sample containing chlorides, once the chlorides precipitate we titrate excess Ag+ with thiocyanate solution in the presence of Fe3+. Once all Ag+ is precipitated excess SCN- creates FeSCN2+ complex with a strong wine color.
Much more interesting is the case of Zn2+ titration with ferrocyanide:
Zn2+ + Fe(CN)64- → Zn2Fe(CN)6
To detetect end point we use rather unexpected indicator - diphenylamine. Diphenylamine is a redox indicator. Before titration, we add to the solution some small amount of ferricyanide. Zinc ferricyanide is not so weakly soluble, so it doesn't interfere with the main precipitation reaction. However, its presence means we have a well known redox system - Fe(CN)64-/Fe(CN)63- - in the solution. As long as we are before equivalence point, concentration of ferrocyanide is very low and redox potential, given by the Nernst equation:
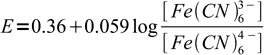 7
7is relatively high. Once we are past the equivalence point concentration of the ferrocyanide goes up, potential goes down, and this difference can be easily detected thanks to the diphenylamine color change.
Adsorption indicators, mentioned earlier, are rarely used, but worth a few words. These are organic molecules, either basic (like rhodamine) or acidic (like fluorescein or eosin). Depending on the titration stage (before or after equivalence point) surface of the precipitate is slightly charged. For example, in the Mohr titration we precipitate AgCl. AgCl has a very large surface due to its colloidal nature. Before the equivalence point there is an excess of chlorides in the solution, and they tend to adsorb on the precipitate surface, charging it negative. After equivalence point there is an excess of Ag+ in the solution and situation changes - surface becomes charged positive. This charge on the surface attracts the organic indicator molecules. Here comes the most important part - these indicators have different colors when they are free in the solution and when they are adsorbed on the precipitate, which helps detect the end point.




