Precipitation titration - equivalence point calculation
At equivalence point we have just a saturated solution of insoluble salt, so calculation of concentration of the determined ion is identical to the solubility calculations. Just for fun, we can derive seriously looking formula that will describe the concentration.
Let's assume we precipitate salt of a general formula MekXl, with known Ksp:
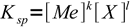 1
1Looking at the formula we can see that
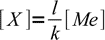 2
2so
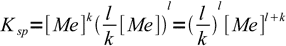 3
3and
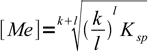 4
4However, in practice you will probably find it easier to solve the problem manually each time, than to look for formula or trying to remember it. That's what we will do in the following example.
Note, that in the real world it is quite often necessary to account for numerous side reactions - especially for protonation and hydrolysis of both metal cation and anion.
Example: calculate pZn at the equivalence point of zinc titration with ferrocyanide, assuming pKsp = 16.8.
We don't need any other information about volumes and concentrations! Let's start with dissolution reaction and Ksp:
Zn2Fe(CN)6 → Zn2+ + Fe(CN)64-
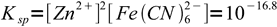 5
5obviously
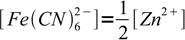 6
6so
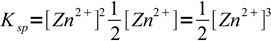 7
7and
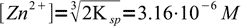 8
8and finally
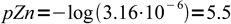 9
9Let's check, if our general formula gives the same result. From the Zn2Fe(CN)6 formula k=2 and l=1, so
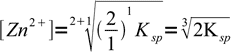 10
10That's the same.




