Burette & pipette - cleaning volumetric glassware used in titrations
Laboratory glassware have to be perfectly clean before it can be used for any type of analytical work. There are two reasons for that. First, you don't want any remaining reagents on the glass surface - they could react with your solutions and change analysis results. Second, if the glass is not perfectly clean, water will not wet its surface, and it will be present on the glass surface in the form of droplets:
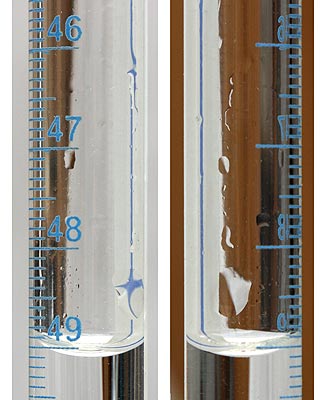
Droplets of the reagent solution on the surface of the greasy glass.
It is impossible to account for volume of these droplets, so you will never know what volume of the reagent was used - and as the precision of the volume measurements is the basis of the precision of the volumetric methods, dirty volumetric glass means huge errors. That's not what we want, thus glass has to be clean.
To check if the glass is clean just fill it with the distilled water and empty it. Water should form a smooth sheet. If the glass was clean and wettable, there will be no droplets visible, if the glass was greasy - they will be present.
It is best to clean the glass immediately after it was used. Most reagents can be easily removed by simple rinsing as long as their solution has not dried on the glass surface. If the solution was concentrated (say stock hydrochloric acid), start with copious amounts of tap water, then rinse with distilled or DI water. If the solution was not concentrated (say 0.1M NaOH titrant) you may rinse it only with with distilled or DI water. Remember to keep the glass in locker when not in use, to avoid air pollution. In the case of burettes we may cover them with paper caps to keep them clean:

Paper cap protecting burette from dust.
Just rinsing will be not always sufficient. Even air dust contains some amount of grease so sooner or later glass surface will become greasy and not wettable. In such cases you will need some kind of glass cleaner. There are many type of these. There are specialized and highly effective detergents for glassware cleaning (like Alconox, LabKlenz, Neodisher and others). Sometimes it is enough to soak the glass in these cleaners (followed by rinsing), sometimes we may need to use a soft brushes (with a wooden or soft plastic handle to avoid abrasion), sometimes use of an ultrasonic cleaner will help. Note, that brushes should be not used for cleaning of pipettes and burettes, as forcing them inside can easily damage precise and expensive glassware.
In some specific cases it can be worth to soak the glass in other reagents - like concentrated hydrochloric acid to clean the glass stained after being used for permanganometric titration, or some organic solvent to remove water insoluble organic compounds.
There are also cleaning solutions that can be prepared in the lab. These are usually highly corrosive and based on strong oxidizing agents (Piranha solution, chromic acid solution) or strong hydrolyzing agents (NaOH ethanol solution). They should be used with care as they can be dangerous, especially Piranha solution can be unpredictable and explode in the presence of organic contaminants. In some cases they can also damage graduation markings on the glass.
- Chromic acid - dissolve slowly 15 g of sodium dichromate in 500 mL of concentrated sulfuric acid. Use gloves, glasses and fume hood. Make sure chromic acid will be in contact only with glass - not with any metal or rubber elements. Make sure glassware doesn't contain water (or at least contains as small amounts of water as possible) before using the mixture. It is best to fill the vessel (do not mouth pipette the solution - use rubber pump!) or soak the item in the solution for a 5-10 minutes. After use put the solution back into bottle and rinse the glass. Initially mixture is dark brown (Cr2O72-). When used it becomes slightly green (Cr3+). When the green tint becomes strong, mixture is spent and no longer active. Make sure spent chromic acid is diluted after use and disposed of properly.
- KOH methanol solution - dissolve 50 g of solid KOH pellets in 500 mL of methanol. Use similarly to chromic acid, with similar precautions. Solution is strongly caustic and can etch the glass, thus it is better to avoid its use to cleaning volumetric glassware.
After cleaning with a detergent or special solution, rinse the glass thoroughly with a tap water. After that rinse with distilled or DI water.
When rinsing with distilled or DI water, rinse 3-4 times. Each rinsing dilutes whatever was present on the glass, so it is better to rinse three times with small volumes of DI water, then to rinse once with a large volume (this is similar to basic idea behind the extraction technique). Be sure you have rinsed whole glass surface.
Don't dry the glass surface with towels, just leave it protected from the dust. It is not necessary to dry the glass in the lab dryer, but if you have one - use it. Not only will it dry the glass faster, but it will also keep the glass protected from the dust during drying.




